Quantum mechanics, which rules the world of the teensy-tiny, may help explain why genetic mutations spontaneously crop up in DNA as it makes copies of itself, a recent study suggests.
Quantum mechanics describes the strange rules that govern atoms and their subatomic components. When the rules of classical physics, which describe the big world, break down, quantum comes in to explain. In the case of DNA, classical physics offers one explanation for why changes can suddenly appear in a single rung of the spiralling ladder of DNA, resulting in what’s called a point mutation.
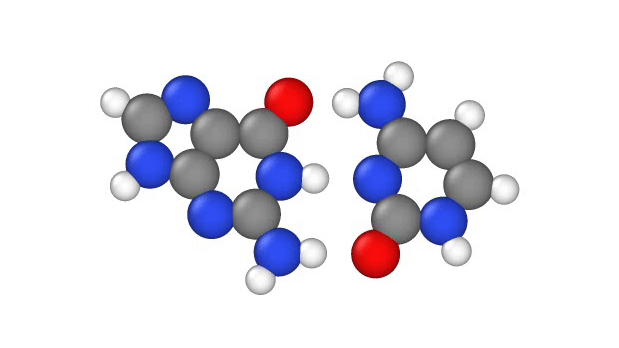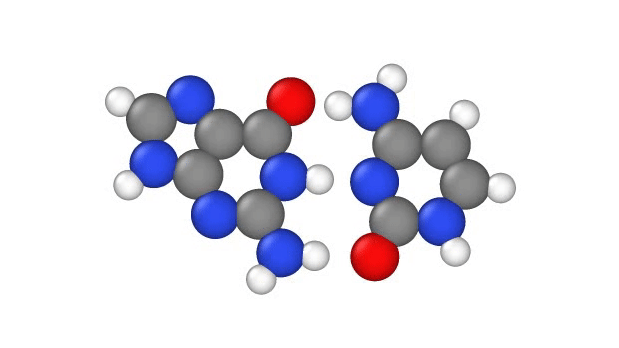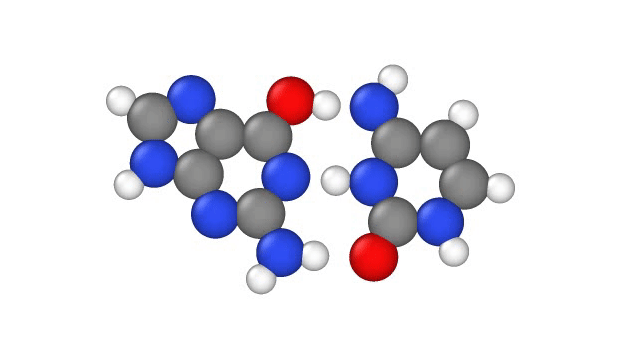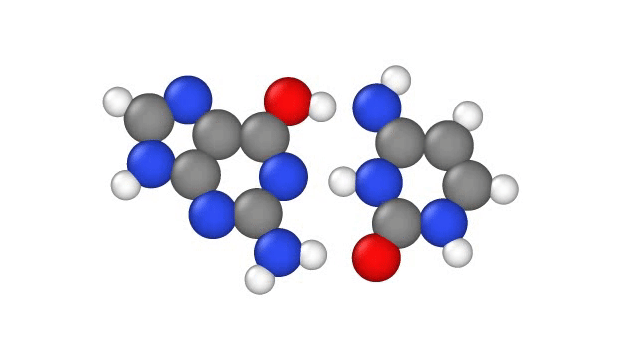
In a recent study, published Jan. 29 in the journal Physical Chemistry Chemical Physics, researchers explore another explanation, showing that a quantum phenomenon called proton tunnelling can cause point mutations by allowing positively charged protons in DNA to leap from place to place. This, in turn, can subtly change the hydrogen bridges that bind the two sides of DNA’s double helix, which can lead to errors when it’s time for DNA to make copies of itself.
In particular, this subtle change can potentially cause misprints in the DNA sequence, where the wrong “letters” get paired together as the strand replicates, the study authors note. These letters, known as bases, usually pair up in a certain way: A to T and G to C. But proton tunneling could cause some bases to mix-and-match.
“There has been quite a lot of computational work looking at hydrogen bonding [and] proton transfer in DNA base pairs,” said Sam Hay, a professor of computational and theoretical chemistry at the University of Manchester, who was not involved in the study. “This paper uses quite high-level calculations to reexamine this phenomenon,” he told Live Science in an email.
However, due to the calculations used, the authors could model only small portions of a DNA strand, at the level of single bases and base pairs. That means the model doesn’t include the two sides of the DNA double-helix, nor the pairs located elsewhere in the strand, Hay noted. These nearby structures may have a “significant effect” on how proton tunneling unfolds, but to model the entire DNA strand would have required an enormous amount of computational power, he said.
“We may have to wait until computing power or methodology improves further before this can be addressed,” he said.
Classical versus quantum
Now, classical physics also provides an explanation for why protons jump around in DNA.
DNA base pairs are joined in the middle by hydrogen bonds — a relatively weak attraction between hydrogen atoms and molecules in the bases. These bonds can be broken by heat, because as the temperature rises, the molecules vigorously vibrate and jiggle, causing the hydrogen atoms to pop out of place.
“You can think of the entire environment jiggling, vibrating … everything is dynamic and moving,” said study co-author Louie Slocombe, a doctoral student at the University of Surrey’s Leverhulme Quantum Biology Doctoral Training Centre in England. Atoms wiggle at any temperature above absolute zero because heat drives up their kinetic energy, or motion, he said.
According to classical thermodynamics, this jiggling sometimes allows hydrogen atoms to jump into new positions in the DNA, briefly forging new bonds. But the atoms soon bounce back to their original locations; due to the molecular structure of DNA bases, hydrogen atoms tend to settle into a somewhat “stable” position between the pairs, where they spend most of their time, and only briefly escape to unusual, “unstable” positions.
Hydrogen atoms contain just one proton, one negatively-charged electron and no neutrons; during the formation of DNA, these atoms “lose” their electron to one base in the pair when they form a bond. So in effect, when hydrogen atoms leap from one side of a DNA strand to the other, they move as a single proton, hence scientists refer to the phenomenon as “proton transfer,” according to a 2014 report in the journal Accounts of Chemical Research.
But according to the new study, classical proton transfer does not account for all the instances that protons bounce around in DNA.
“Essentially, what we find is that the amount of this [happening] just via classical thermodynamics is very low, in comparison to when we run the numbers for quantum rates,” Slocombe said. In other words, proton tunneling likely drives more proton-jumping than heat alone does, he said.
To Read More: Click Here
References:
Nicoletta Lanese (March 16, 2021). Why does DNA spontaneously mutate? Quantum physics might explain. LiveScience. Retrieved from https://buff.ly/3rVNp2H